Abstract
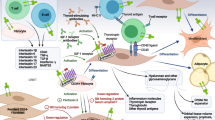
Graves’ ophthalmopathy (GO) is an autoimmune orbital disorder most commonly associated with Graves’ disease. Recent studies have underscored the role that orbital cells, particularly fibroblasts and adipocytes, play in causing the increase in orbital content responsible for clinical manifestations of the disease. GO seems to be related to autoimmune reactions triggered by autoreactive T lymphocytes of thyroid origin, which recognize antigen(s) shared by thyroid and orbit. The nature of the antigen (or antigens) involved is not fully understood, but TSH receptor is likely to be involved. Cytokines secreted by T lymphocytes, macrophages and fibroblasts play an essential role in perpetuating the disease. Animal models of GO have been developed, but results have not clarified GO pathogenesis yet. Progress in the management of the ophthalmopathy has been very limited, and glucocorticoids, orbital radiotherapy and orbital decompression remain the mainstays in GO treatment. Novel treatments, such as somatostatin analogues, antioxidants, cytokine antagonists are currently under investigation, as well as the effects of total thyroid ablation. Cessation of smoking currently represents the only form of GO (secondary and tertiary) prevention.
Similar content being viewed by others
References
Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev 2000, 21: 168–99.
Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid 2002, 12: 855–60.
Bartalena L, Marcocci C, Pinchera A. Graves’ ophthalmopathy: a preventable disease? Eur J Endocrinol 2002, 146: 457–61.
Bartalena L, Bogazzi F, Tanda ML, Manetti L, Dell’Unto E, Martino E. Cigarette smoking and the thyroid. Eur J Endocrinol 1995, 133: 507–12.
Bartalena L. Smoking and Graves’ disease. J Endocrinol Invest 2002, 25: 402.
Hegedus L, Brix TH, Vestergaard P. Relation between cigarette smoking and Graves’ ophthalmopathy. J Endocrinol Invest 2004, 27: 265–71.
Gerding MN, Terwee CB, Dekker FW, Koornneef L, Prummel MF, Wiersinga WM. Quality of life in patients with Graves’ ophthalmopathy is markedly decreased: measurement by the Medical Outcomes Study Instrument. Thyroid 1997, 7: 885–9.
Bartley GB, Fatourechi V, Kardmas EF, et al. Long-term follow-up of Graves’ ophthalmopathy in an incidence cohort. Ophthalmology 1996, 103: 958–62.
Ludgate M, Baker G. Inducing Graves’ ophthalmopathy. J Endocrinol Invest 2004, 27: 211–5.
Costagliola S, Many MC, Stalmans Falys M, et al. Transfer of thyroiditis, with syngeneic spleen-cells sensitized with the human thyrotropin receptor, to naive BALB/c and NOD mice. Endocrinology 1996, 137: 4637–43.
Many MC, Costagliola S, Detrait M, Denef JF, Vassart G, Ludgate M. Development of an animal model of autoimmune thyroid eye disease. J Immunol 1999, 162: 4966–74.
Costagliola S, Many MC, Denef JF, Pohlenz J, Refetoff S, Vassart G. Genetic immunisation of outbred mice with thyrotropin receptor cDNA provides a model of Graves’ disease. J Clin Invest 2000, 105: 803–11.
Coles AJ, Wing N, Smith S, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 1999, 354: 1691–5.
Bahn RS. TSH receptor expression in orbital tissue and its role in the pathogenesis of Graves’ ophthalmopathy. J Endocrinol Invest 2004, 27: 216–20.
Davies TF. The thyrotropin receptors spread themselves around. J Clin Endocrinol Metab 1994, 79: 1232–3.
Marinò M, Chiovato L, Lisi S, Altea MA, Marcocci C, Pinchera A. Role of thyroglobulin in the pathogenesis of Graves’ ophthalmopathy: the hypothesis of Kriss revisited. J Endocrinol Invest 2004, 27: 230–6.
McDougall IR, Kriss JP. New thoughts about the case and treatment of the severe ocular manifestations of Graves’ disease. Scott Med J. 1974, 19: 165–9.
Marinò M, Lisi S, Pinchera A, et al. Identification of thyroglobulin in orbital tissues of patients with thyroid-associated ophthalmopathy. Thyroid 2001, 11: 177–85.
Lisi S, Marinò M, Pinchera A, et al. Thyroglobulin in orbital tissues from patients with thyroid associated ophthalmopathy: predominant localization in fibroadipose tissue. Thyroid 2002, 12: 351–60.
Mariotti S, Pisani S, Russova A, Pinchera A. A new solid-phase immunoradiometric assay for anti-thyroglobulin autoantibody. J Endocrinol Invest 1982, 5: 27–33.
Rose NR. Differing responses of inbred rat strains in experimental autoimmune thyroiditis. Cell Immunol 1975, 18: 360–4.
Mikozami T, Salvi M, Wall JR. Eye muscle antibodies in Graves’ ophthalmopathy: pathogenic or secondary epiphenomenon? J Endocrinol Invest 2004, 27: 221–9.
Hiromatsu Y, Fukazawa H, Guinard F, Salvi M, How J, Wall JR. A thyroid cytotoxic antibody that cross-reacts with an eye muscle cell surface antigen may be the cause of thyroidassociated ophthalmopathy. J Clin Endocrinol Metab 1988, 67: 565–70.
Kubota S, Gunji K, Stolarski C, Kennerdell JS, Wall JR. Role of eye muscle antibody measurement in diagnosis of thyroid-associated ophthalmopathy: a laboratory update. Endocr Prac 1998, 4: 127–31.
Ajjan RA, Weetman AP. New understanding of the role of cytokines in the pathogenesis of Graves’ ophthalmopathy. J Endocrinol Invest 2004, 27: 237–45.
Heufelder AE, Bahn R. Detection and localization of cytokine immunoreactivity in retro-ocular connective tissue in Graves’ ophthalmopathy. Eur J Clin Invest 1993, 23: 10–7.
McLachlan SM, Prummel MF, Rapoport B. Cell-mediated or humoral immunity in Graves’ ophthalmopathy? Profiles of T-cell cytokines amplifies by polymerase chain reaction from orbital tissue. J Clin Endocrinol Metab 1994, 78: 1070–4.
Hiromatsu Y, Yang D, Bednarczuk T, Mayake I, No K, Inoue Y. Cytokine profile in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2000, 85: 1194–9.
Heufelder AE, Smith J, Gorman CA, Bahn RS. Increased induction of HLA-DR by interferon-γ in cultured retroocular fibroblasts from patients with Graves’ ophthalmopathy and pretibial myxedema. J Clin Endocrinol Metab 1991, 73: 307–13.
Heufelder AE. Pathogenesis of Graves’ ophthalmopathy: recent controversies and progress. Eur J Endocrinol 1995, 132: 532–41.
Heufelder AE, Bahn RS. Modulation of intercellular adhesion molecule-1 (ICAM-1) by cytokines and Graves’ Igs in cultured Graves’ retroocular fibroblasts. Eur J Clin Invest 1992, 23: 10–7.
Heufelder AE, Bahn RS. Modulation of Graves’ orbital fibroblast proliferation by cytokines and glucocorticoid receptor agonists. Ophthalmol Vis Sci 1994, 35: 120–7.
Ajjan RA, Weetman AP. Cytokines in Graves’ disease. In: Rapoport B, McLachlan S eds. Graves’ disease: pathogenesis and treatment. Norwell: Kluiwer Academic Publ. 2000, 79–93.
Cao HJ, Smith TJ. Leukoregulin upregulation of prostaglandin endoperoxide H synthase-2 expression in human orbital fibroblasts. Am J Physiol 1999, 277: 1075–85.
Sorisky A, Pardasani D, Gagnon A, Smith TJ. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab 1996, 81: 3428–31.
Smith TJ. Novel aspects of orbital fibroblast pathology. J Endocrinol Invest 2004, 27: 246–53.
Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity 1993, 16: 251–7.
Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor- γ (PPAR- γ), and thyrotropin receptor by PPAR- γ agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab 2002, 87: 2352–8.
Starkey K, Heufelder AE, Baker G, et al. PPAR γ in thyroid eye disease: contraindication to thiazolidenedione use? J Clin Endocrinol Metab 2003, 88: 55–9.
Bartalena L, Tanda ML, Piantanida E, Lai A. Oxidative stress and Graves’ ophthalmopathy: in vitro studies and therapeutic implications. Biofactors 2003, 19: 155–63.
Burch HB, Lahiri S, Bahn RS, Barnes S. Superoxide radical production stimulates retroocular fibroblast proliferation in Graves’ ophthalmopathy. Exp Eye Res 1997, 65: 311–6.
Hiromatsu Y, Yang D, Miyake I, et al. Nicotinamide decreases cytokine-induced activation of orbital fibroblasts from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 1998, 83: 121–4.
Lu R, Wang P, Wartofsky L, et al. Oxygen free radicals in interleukin-1β-induced glycosaminoglycan production by retro-ocular fibroblasts from normal subjects and Graves’ ophthalmopathy patients. Thyroid 1999, 9: 297–303.
Heufelder AE, Wenzel BE, Bahn RS. Methimazole and propylthiouracil inhibit the oxygen free radical-induced expression of a 72 kilodalton heat shock protein in Graves’ retroocular fibroblasts. J Clin Endocrinol Metab 1992, 73: 307–13.
Hiromatsu Y, Sato M, Yamada K, Nonaka K. Nicotinamide and 3-amidobenzamide inhibit recombinant interferon- γ-induced HLA-DR antigen expression, but not HLA-A, B, C antigen expression, on cultured human thyroid cells. Clin Endocrinol (Oxf) 1992, 36: 91–5.
Hiromatsu Y, Sato M, Tanaka K, Ishisaka N, Kamachi J, Nonaka K. Inhibitory effects of nicotinamide on intercellular adhesion molecule-1 expression on cultured human thyroid cells. Immunology 1993, 80: 330–2.
Bartalena L, Marcocci C, Pinchera A. Cytokine antagonists: new ideas for the management of Graves’ ophthalmopathy. J Clin Endocrinol Metab 1996, 81: 446–8.
Tan GH, Dutton CM, Bahn RS. Interleukin-1 (IL-1) receptor antagonist and soluble IL-1 receptor inhibit IL-1-induced glycosaminoglycan production in cultured human orbital fibroblasts from patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab 1996, 81: 449–52.
Marcocci C, Marinò M, Rocchi R, Menconi F, Morabito E, Pinchera A. Novel aspects of immunosuppressive and radiotherapy management of Graves’ ophthalmopathy. J Endocrinol Invest 2004, 27: 272–80.
Marcocci C, Bartalena L, Tanda ML, et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab 2001, 86: 3562–7.
Bartalena L, Marcocci C, Tanda ML, Pinchera A. Management of thyroid eye disease. Eur J Nucl Med 2002, 29 (Suppl. 2): S458–65.
Bartalena L, Marcocci C, Gorman CA, Wiersinga WM, Pinchera A. Orbital radiotherapy for Graves’ ophthalmopathy: useful or useless? Safe or dangerous? J Endocrinol Invest 2003, 26: 5–18.
Krassas GE. Somatostatin analogues: a new tool for management of Graves’ ophthalmopathy. J Endocrinol Invest 2004, 27: 281–7.
Krassas GE, Heufelder AE. Immunosuppressive therapy in patients with thyroid eye disease: an overview of current concepts. Eur J Endocrinol 2001, 144: 311–8.
Bartalena L, Tanda ML, Piantanida E, Lai A. The role of somatostatin analogs in the management of Graves’ ophthalmopathy? J Endocrinol Invest 2003, 26 (Suppl to no. 8): 109–13.
Kendall-Taylor P, Dickinson AJ, Vaidya B, et al. Double-blind placebo-controlled trial of octreotide LAR in thyroid-associated orbitopathy (TAO): clinical outcomes. Thyroid 2003, 15: 671 (abstract).
Prummel MF, Mourits MP, Berghout A, et al. Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N Engl J Med 1989, 321: 1353–9.
Smith JR, Rosenbaum JT. A role of methotrexate in the management of non-infectious orbital inflammatory disease. Br J Ophthalmol 2001, 85: 1220–4.
Bouzas EA, Karadimas P, Mastorakos G, Koutras DA. Antioxidants agents in the treatment of Graves’ ophthalmopathy. Am J Ophthalmol 2000, 129: 618–22.
Balazs C, Kiss E, Vamos A, Molnar I, Farid NR. Beneficial effect of pentoxifylline on thyroid associated ophthalmopathy (TAO): a pilot study. J Clin Endocrinol Metab 1997, 82: 1999–2002.
Bartalena L, Tanda ML, Medea A, Marcocci C, Pinchera A. Novel approaches to the management of Graves’ ophthalmopathy. Hormones 2002, 1: 76–90.
Bartalena L, Tanda ML, Piantanida E, Lai A, Pinchera A. Relationship between management of hyperthyroidism and course of the ophthalmopathy. J Endocrinol Invest 2004, 27: 288–94.
Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med 1998, 338: 73–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartalena, L., Wiersinga, W.M. & Pinchera, A. Graves’ ophthalmopathy: State of the art and perspectives. J Endocrinol Invest 27, 295–301 (2004). https://doi.org/10.1007/BF03345280
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345280